Torecan Prescribing Information
Package insert / product label
Generic name: thiethylperazine maleate
Dosage form: Tablets, USP and Injection, USP
Drug class: Phenothiazine antiemetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
T1999-31
89007701
TORECAN®
(thiethylperazine maleate)
Tablets, USP
(thiethylperazine malate)
Injection, USP
(for intramuscular use only)
Prescribing Information
Rx only
Torecan Description
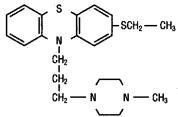
TORECAN® (thiethylperazine) is a phenothiazine. Thiethylperazine is characterized by a substituted thioethyl group at position 2 in the phenothiazine nucleus, and a piperazine moiety in the side chain. The chemical designation is: 2-ethyl-mercapto-10-[3’- (1”-methyl-piperazinyl-4”)-propyl-1’] phenothiazine. Thiethylperazine has the following structural formula:
Tablet, 10 mg, for oral administration
Active Ingredient: thiethylperazine maleate USP, 10 mg.
Inactive Ingredients: acacia, carnauba wax, FD&C Yellow No. 5 aluminum lake (tartrazine), FD&C Yellow No. 6 aluminum lake, gelatin, lactose, magnesium stearate, povidone, sodium benzoate, sorbitol, starch, stearic acid, sucrose, talc, titanium dioxide.
Related/similar drugs
ondansetron, hydroxyzine, lorazepam, dexamethasone, olanzapine, promethazine
ACTIONS
The pharmacodynamic action of TORECAN® (thiethylperazine) in humans is unknown. However, a direct action of TORECAN® (thiethylperazine) on both the CTZ and the vomiting center may be concluded from induced vomiting experiments in animals.
Indications and Usage for Torecan
TORECAN® (thiethylperazine) is indicated for the relief of nausea and vomiting.
Contraindications
Severe central nervous system (CNS) depression and comatose states.
Use of TORECAN® (thiethylperazine) is contraindicated in patients who have demonstrated a hypersensitivity reaction (e.g., blood dyscrasias, jaundice) to phenothiazines.
Because severe hypotension has been reported after the intravenous administration of phenothiazines, this route of administration is contraindicated.
Warnings
TORECAN® (thiethylperazine) Injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Phenothiazines are capable of potentiating CNS depressants (e.g., barbiturates, anesthetics, opiates, alcohol, etc.) as well as atropine and phosphorous insecticides.
Since TORECAN® (thiethylperazine) may impair mental and/or physical ability required in the performance of potentially hazardous tasks such as driving a car or operating machinery, it is recommended that patients be warned accordingly.
Postoperative Nausea and Vomiting
With the use of this drug to control postoperative nausea and vomiting occurring in patients undergoing elective surgical procedures, restlessness and postoperative CNS depression during anesthesia recovery may occur. Possible postoperative complications of a severe degree of any of the known reactions of this class of drug must be considered. Postural hypotension may occur after an initial injection, rarely with the tablet.
The administration of epinephrine should be avoided in the treatment of drug-induced hypotension in view of the fact that phenothiazines may induce a reversed epinephrine effect on occasion.
Should a vasoconstrictive agent be required, the most suitable are norepinephrine bitartrate and phenylephrine.
The use of this drug has not been studied following intracardiac and intracranial surgery.
Precautions
Abnormal movements such as extrapyramidal symptoms (E.P.S.) (e.g., dystonia, torticollis, dysphasia, oculogyric crises, akathisia) have occurred. Convulsions have also been reported. The varied symptom complex is more likely to occur in young adults and children. Extrapyramidal effects must be treated by reduction of dosage or cessation of medication.
TORECAN® (thiethylperazine) tablets contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Use in patients with bone marrow depression only when potential benefits outweigh risks.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with phenothiazine drugs. Clinical manifestations include: hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability.
The extrapyramidal symptoms which can occur secondary to TORECAN® (thiethylperazine) may be confused with the central nervous system signs of an undiagnosed primary disease responsible for the vomiting, e.g., Reye’s Syndrome or other encephalopathy. The use of TORECAN® (thiethylperazine) and other potential hepatotoxins should be avoided in children and adolescents whose signs and symptoms suggest Reye’s Syndrome.
Phenothiazine drugs may cause elevated prolactin levels that persist during chronic administration. Since approximately one-third of human breast cancers are prolactin-dependent in vitro, this elevation is of potential importance if phenothiazine drug administration is contemplated in a patient with a previously-detected breast cancer. Neither clinical nor epidemiologic studies to date, however, have shown an association between the chronic administration of phenothiazine drugs and mammary tumorigenesis.
Postoperative Nausea and Vomiting
When used in the treatment of nausea and/or vomiting associated with anesthesia and surgery, it is recommended that TORECAN® (thiethylperazine) should be administered by deep intramuscular injection at or shortly before the termination of anesthesia.
Information for Patients
Patients receiving TORECAN® (thiethylperazine) should be cautioned about possible combined effects with alcohol and other CNS depressants. Patients should be cautioned not to operate machinery or drive a motor vehicle after ingesting the drug.
Drug Interactions
Phenothiazines are capable of potentiating CNS depressants (e.g., barbiturates, anesthetics, opiates, alcohol, etc.) as well as atropine and phosphorous insecticides.
Laboratory Test Interactions
The usual precautions should be observed in patients with impaired renal or hepatic function.
Adverse Reactions/Side Effects
Central Nervous System
Serious: Convulsions have been reported. Extrapyramidal symptoms (E.P.S.) may occur, such as dystonia, torticollis, oculogyric crises, akathisia and gait disturbances. Others: Occasional cases of dizziness, headache, fever and restlessness have been reported.
Drowsiness may occur on occasion, following an initial injection. Generally this effect tends to subside with continued therapy or is usually alleviated by a reduction in dosage.
Autonomic Nervous System
Dryness of the mouth and nose, blurred vision, tinnitus. An occasional case of sialorrhea together with altered gustatory sensation has been observed.
Phenothiazine Derivatives
The physician should be aware that the following have occurred with one or more phenothiazines and should be considered whenever one of these drugs is used:
Blood Dyscrasias Serious–Agranulocytosis, leukopenia, thrombocytopenia, aplastic anemia, pancytopenia. Other: Eosinophilia, leukocytosis.
Autonomic Reactions–Miosis, obstipation, anorexia, paralytic ileus.
Cutaneous Reactions Serious–Erythema, exfoliative dermatitis, contact dermatitis.
Hepatotoxicity Serious–Jaundice, biliary stasis.
Cardiovascular Effects Serious–Hypotension, rarely leading to cardiac arrest; electrocardiographic (ECG) changes.
Extrapyramidal Symptoms Serious–Akathisia, agitation, motor restlessness, dystonic reactions, trismus, torticollis, opisthotonos, oculogyric crises, tremor, muscular rigidity, akinesia–some of which have persisted for several months or years especially in patients of advanced age with brain damage.
Endocrine Disturbances–Menstrual irregularities, altered libido, gynecomastia, weight gain. False positive pregnancy tests have been reported.
Urinary Disturbances–Retention, incontinence.
Allergic Reactions Serious–Fever, laryngeal edema, angioneurotic edema, asthma.
Others: Hyperpyrexia. Behavioral effects suggestive of a paradoxical reaction have been reported. These include excitement, bizarre dreams, aggravation of psychoses and toxic confusional states. While there is no evidence at present that ECG changes observed in patients receiving phenothiazines are in any way precursors of any significant disturbance of cardiac rhythm, it should be noted that sudden and unexpected deaths apparently due to cardiac arrest have been reported in a few instances in hospitalized psychotic patients previously showing characteristic ECG changes. A peculiar skin-eye syndrome has also been recognized as a side effect following long-term treatment with certain phenothiazines. This reaction is marked by progressive pigmentation of areas of the skin or conjunctiva and/or accompanied by discoloration of the exposed sclera and cornea. Opacities of the anterior lens and cornea described as irregular or stellate in shape have also been reported.
Overdosage
Manifestations of acute overdosage of TORECAN® (thiethylperazine) can be expected to reflect the CNS effects of the drug and include extrapyramidal symptoms (E.P.S.), confusion and convulsions with reduced or absent reflexes, respiratory depression and hypotension. If the patient is conscious, vomiting should be induced mechanically or with emetics. Gastric lavage should be employed utilizing concurrently a cuffed endotracheal tube if the patient is unconscious to prevent aspiration and pulmonary complications. Maintenance of adequate pulmonary ventilation is essential. The use of pressor agents intravenously may be necessary to combat hypotension. The administration of epinephrine should be avoided since phenothiazines may induce a reversed epinephrine effect. The most suitable vasoconstrictive agents are norepinephrine and phenylephrine. Fluids should be administered intravenously to encourage diuresis. The value of dialysis has not been determined. If excitation occurs, barbiturates should not be used. It should be borne in mind that multiple agents may have been ingested.
Torecan Dosage and Administration
| TORECAN
thiethylperazine maleate tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| TORECAN
thiethylperazine maleate injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Roxane Laboratories, Inc. |
More about Torecan (thiethylperazine)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: phenothiazine antiemetics
- Breastfeeding
